Features
Study Information Management
Easily handle study information, including animal, dosage, and slide details.
Easily handle study information, including animal, dosage, and slide details.
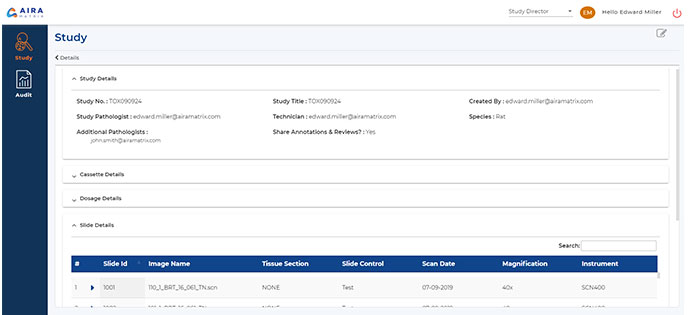
Study Information Management
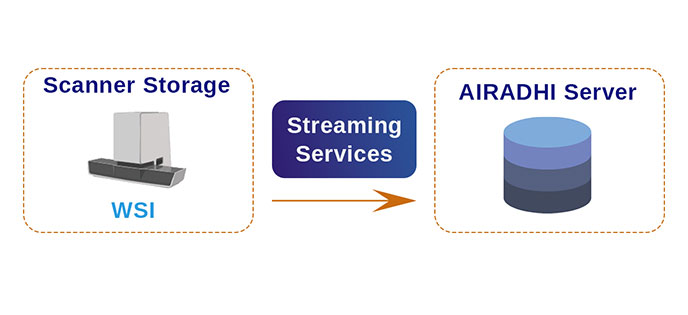
Stream Images Directly
Stream Images Directly
Work directly with the source image, eliminating repetitive uploads and downloads, storage requirements, and delays.
Work directly with the source image, eliminating repetitive uploads and downloads, storage requirements, and delays.
Image and Metadata Mapping
Map images and corresponding metadata to their respective studies for organizational efficiency.
Map images and corresponding metadata to their respective studies for organizational efficiency.
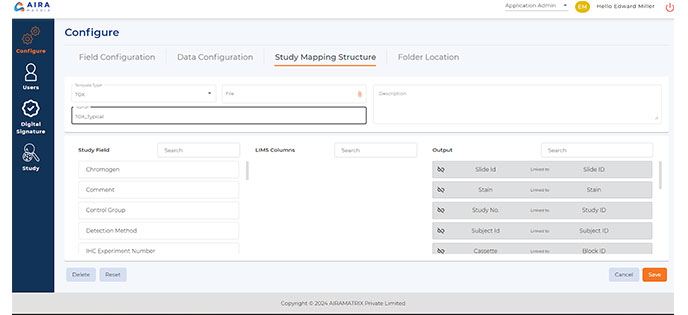
Image and Metadata Mapping
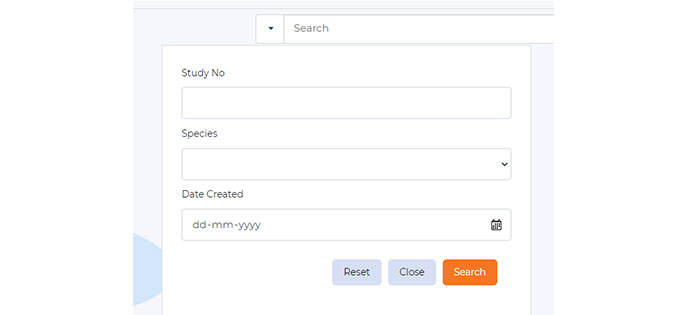
Search and Retrieval
Search and Retrieval
Utilize comprehensive search and filtering to pinpoint the exact image you need
Utilize comprehensive search and filtering to pinpoint the exact image you need
Advanced Viewer Capabilities
Utilize features including slide metadata display, hands-free navigation, annotation tools, fine-tuning, and synced image comparison for ease of viewing and analysis
Utilize features including slide metadata display, hands-free navigation, annotation tools, fine-tuning, and synced image comparison for ease of viewing and analysis
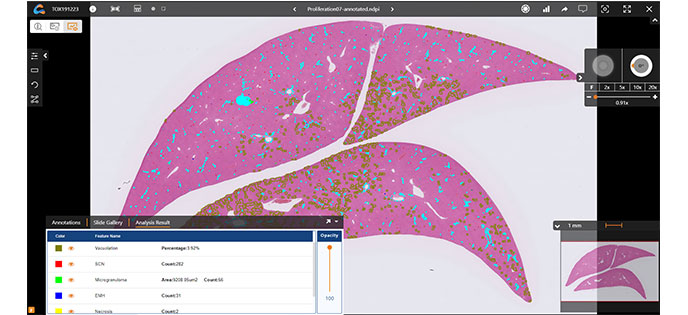
Advanced Viewer Capabilities
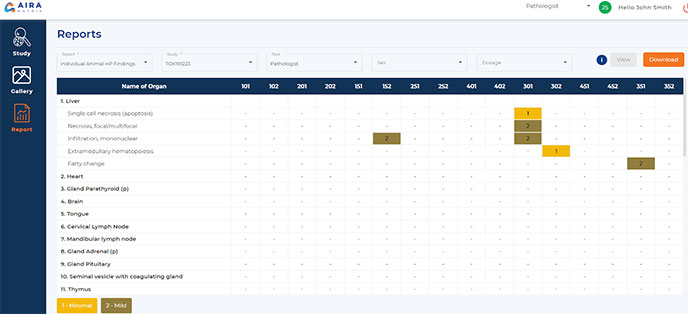
Report Generation
Report Generation
Generate reports with customizable formats for manual or automated image analysis
Generate reports with customizable formats for manual or automated image analysis


